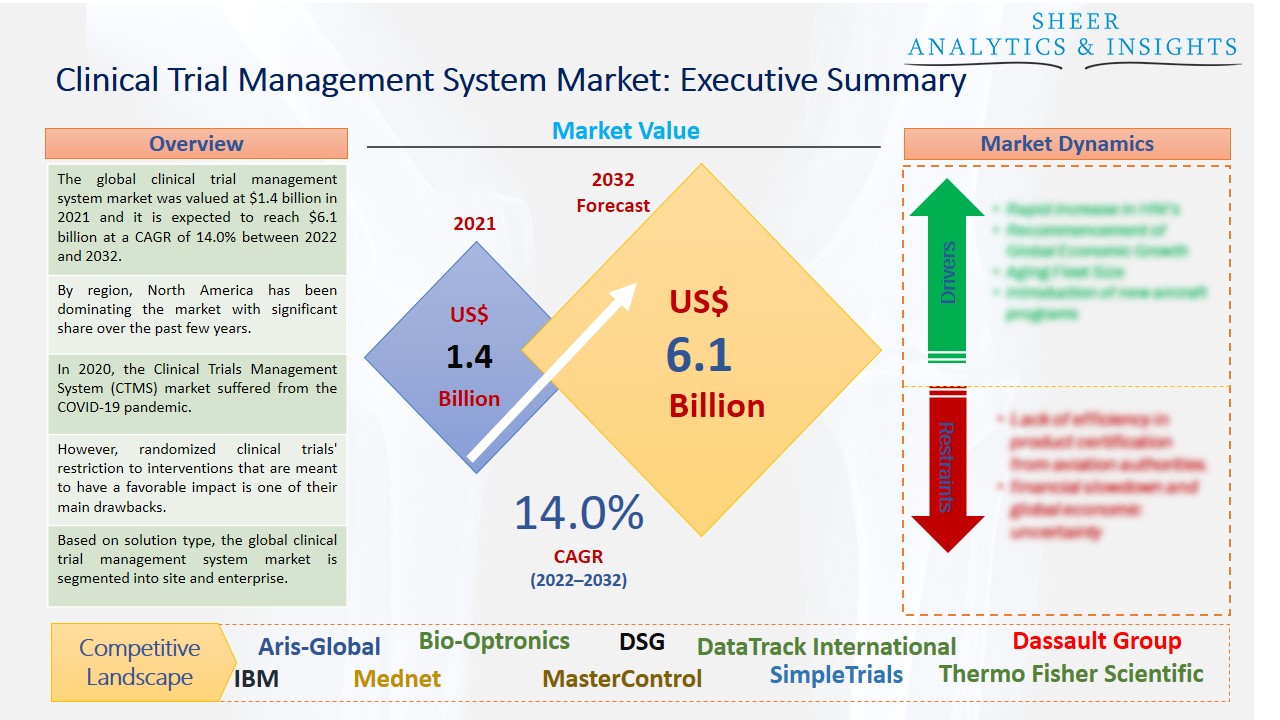
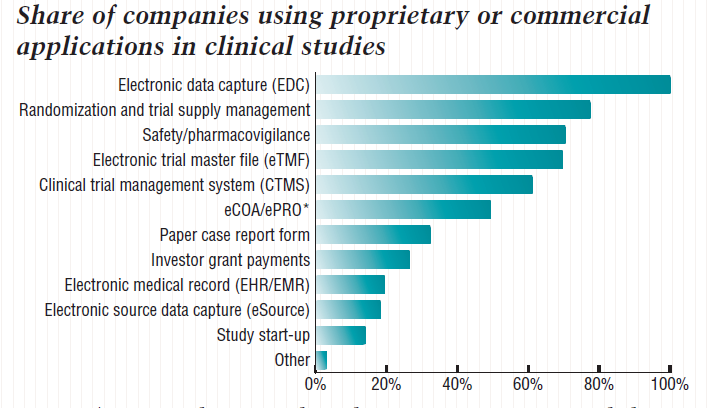
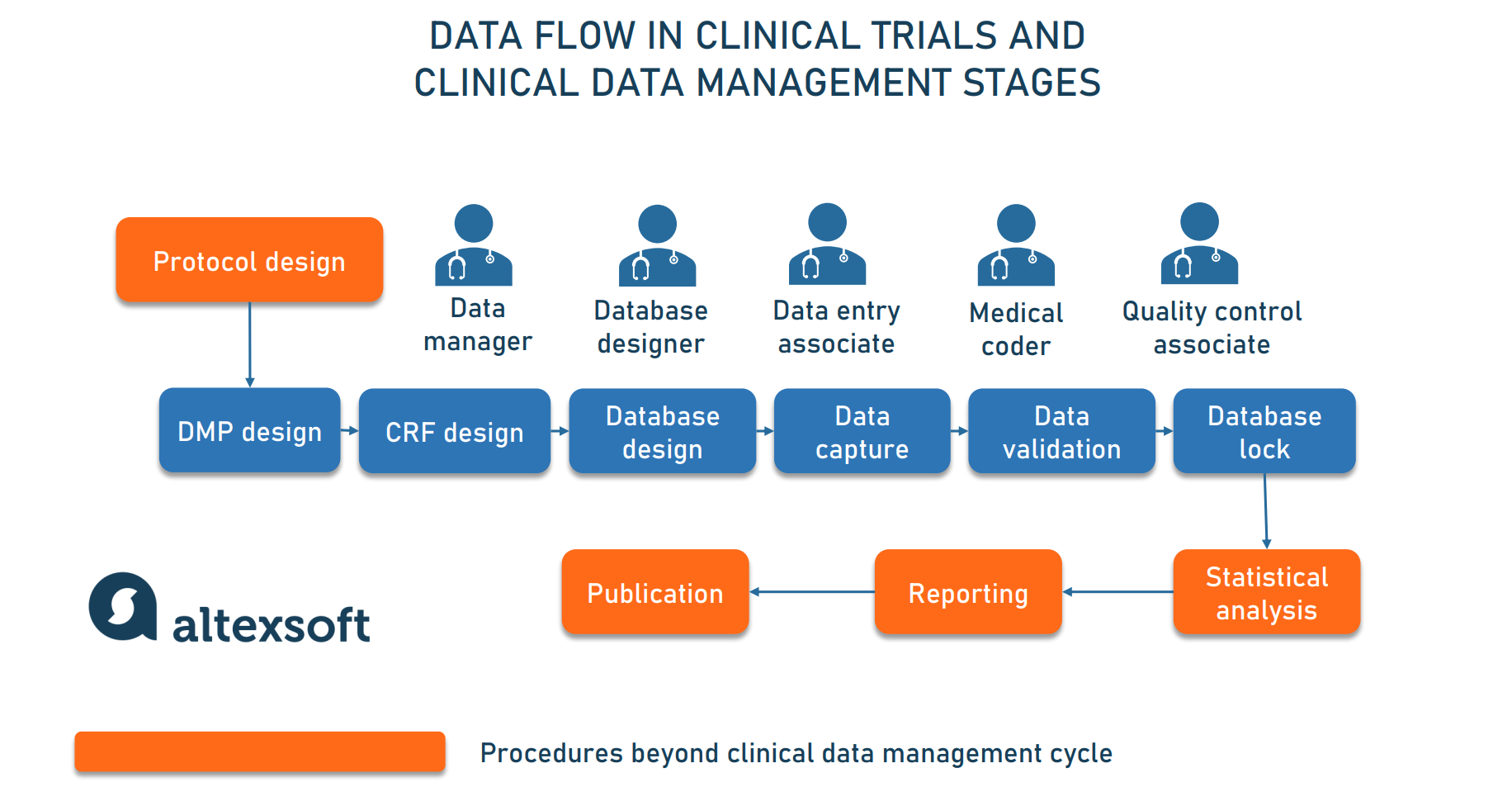
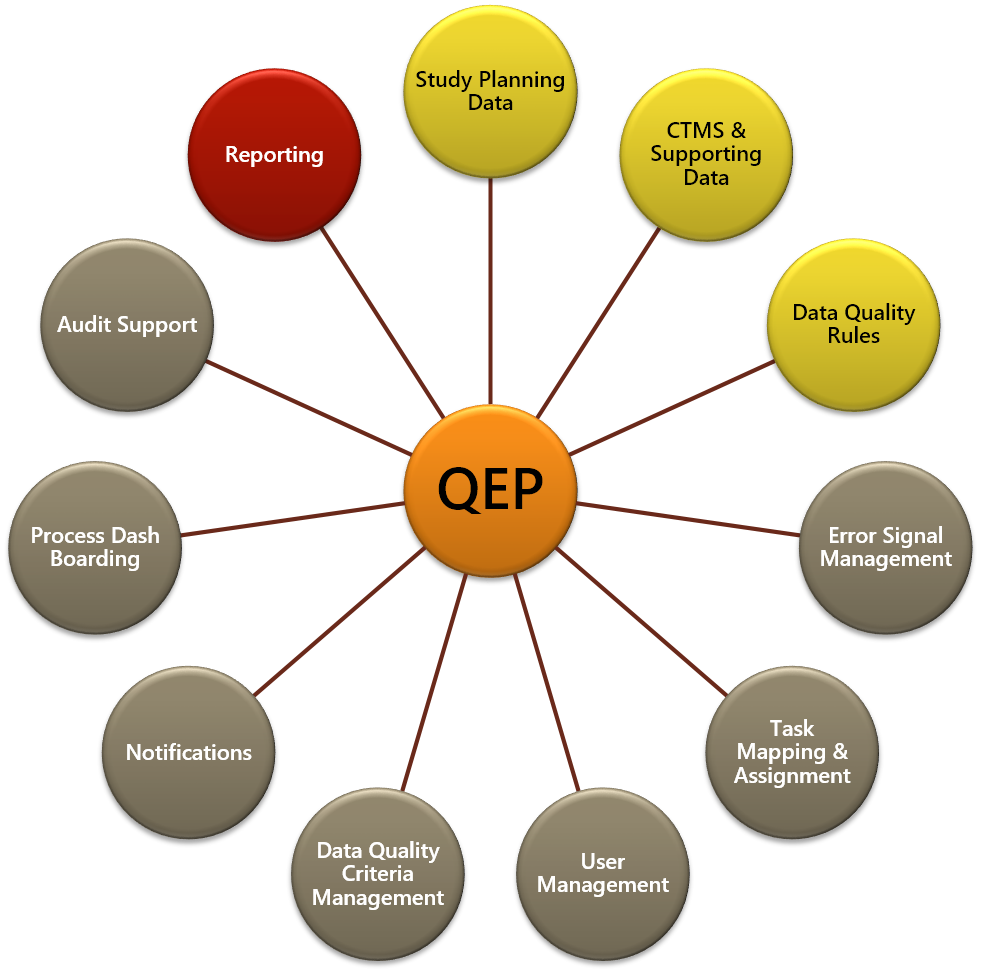
![PDF] Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development | Semantic Scholar PDF] Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b6a9ea604d7a322a539e1b7894536e2da07ce6a6/7-Figure2-1.png)
PDF] Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development | Semantic Scholar
![PDF] A database system for integrated clinical trial management, control, statistical analysis and ICH-compliant reporting | Semantic Scholar PDF] A database system for integrated clinical trial management, control, statistical analysis and ICH-compliant reporting | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b7222080945658c3980e16ccdbbceead72486f32/2-Figure1-1.png)
PDF] A database system for integrated clinical trial management, control, statistical analysis and ICH-compliant reporting | Semantic Scholar

PDF) On selecting a clinical trial management system for large scale, multi-centre, multi-modal clinical research study
A new risk and issue management system to improve productivity, quality, and compliance in clinical trials. - Document - Gale Academic OneFile

Clinical Trial Management System Market by Product, Application, End Users - Forecast By 2030 by marketindustryreports - Issuu

About PennCTMS | Office of Clinical Research | Perelman School of Medicine at the University of Pennsylvania

PDF) Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development

PDF) Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development
A Time and Cost Effective Data Management System for Clinical Research Data to Support Clinical Monitoring Guidelines - Madeline C. van Hoose, Floyd E. Leaders, 1980