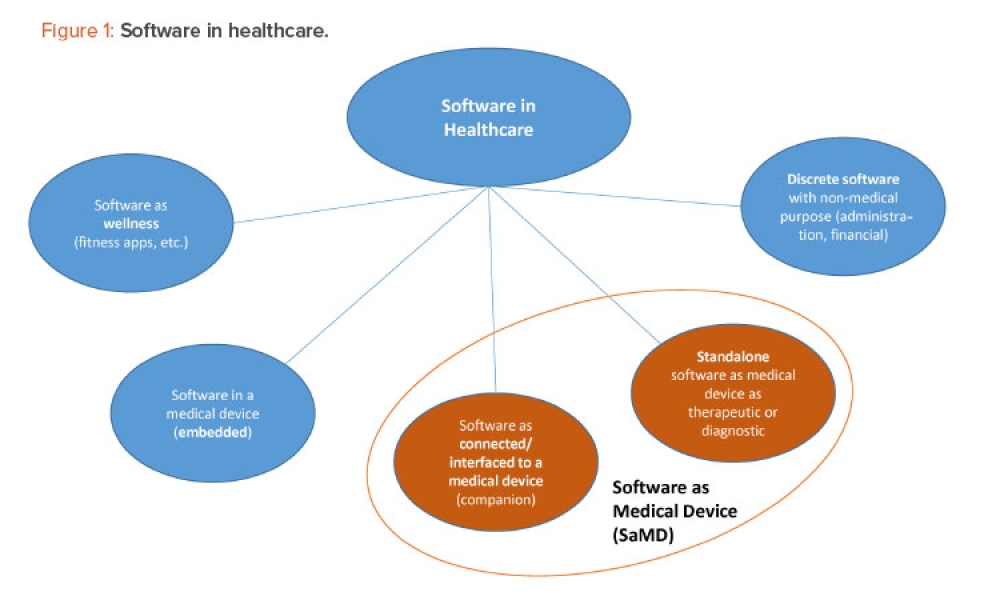
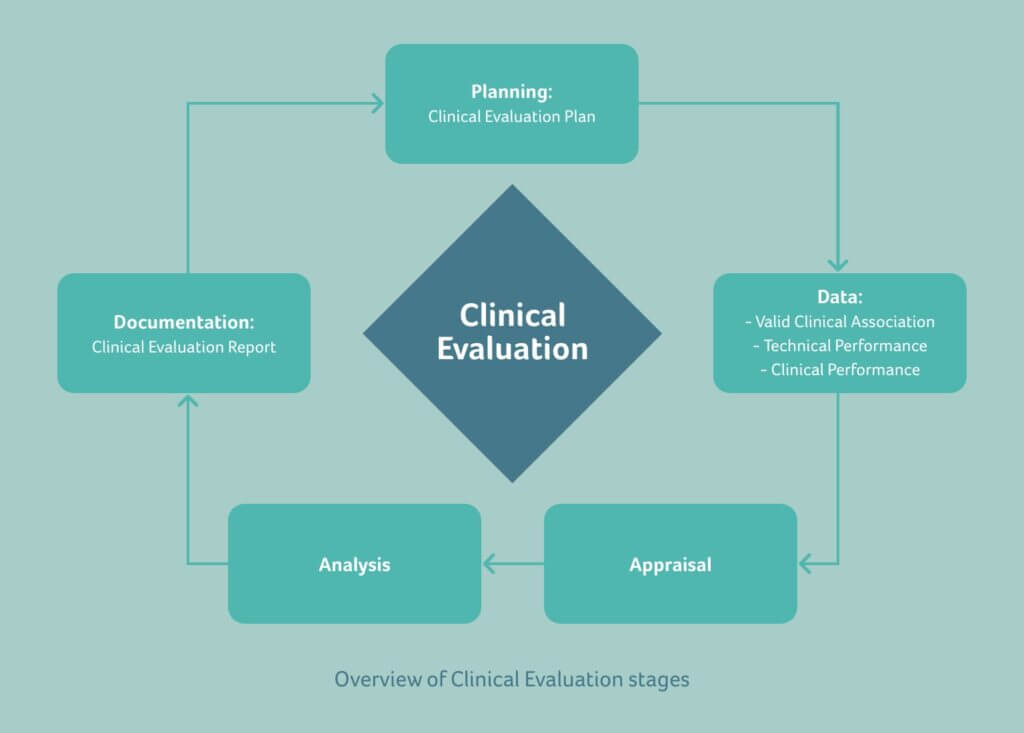
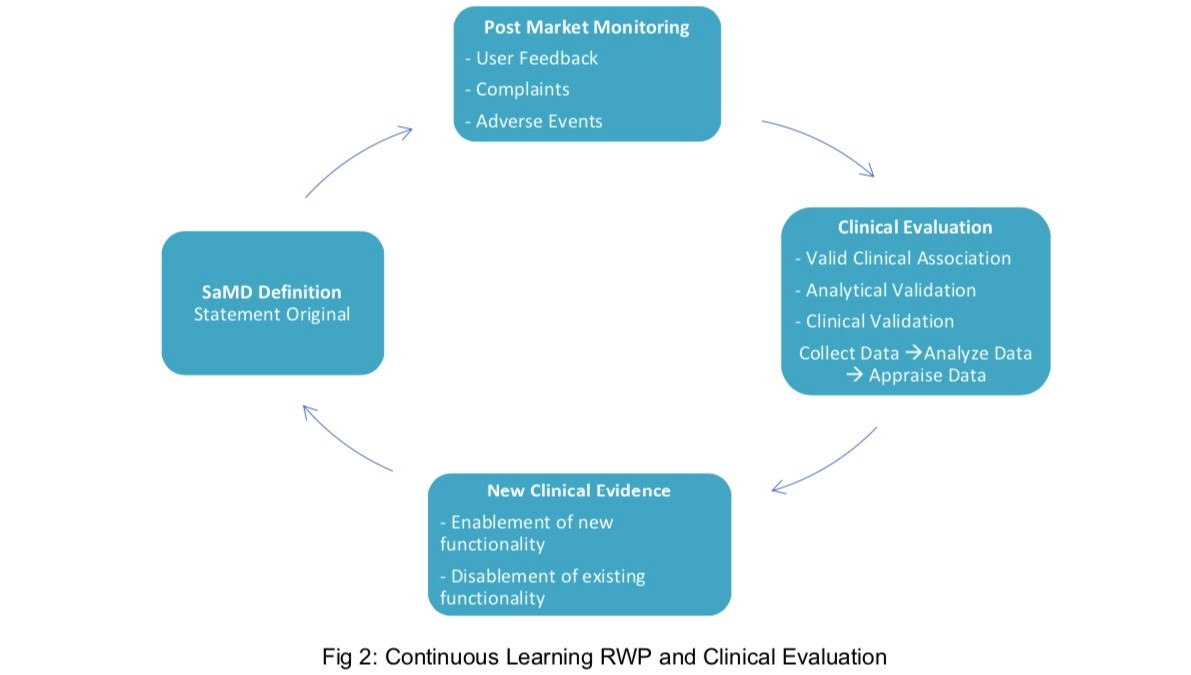
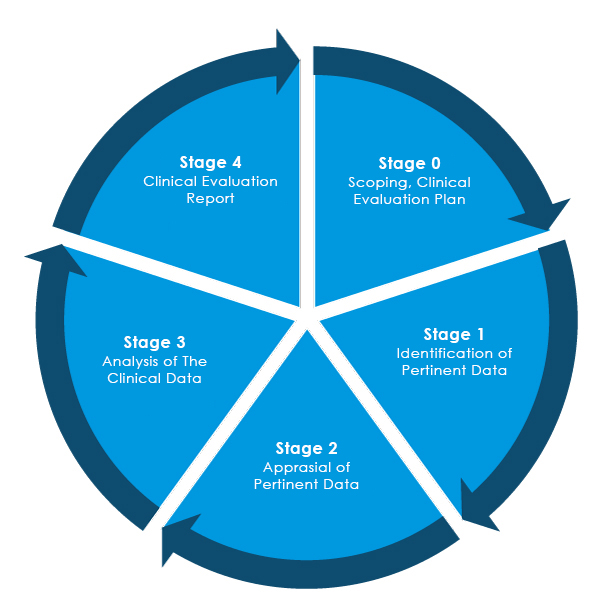
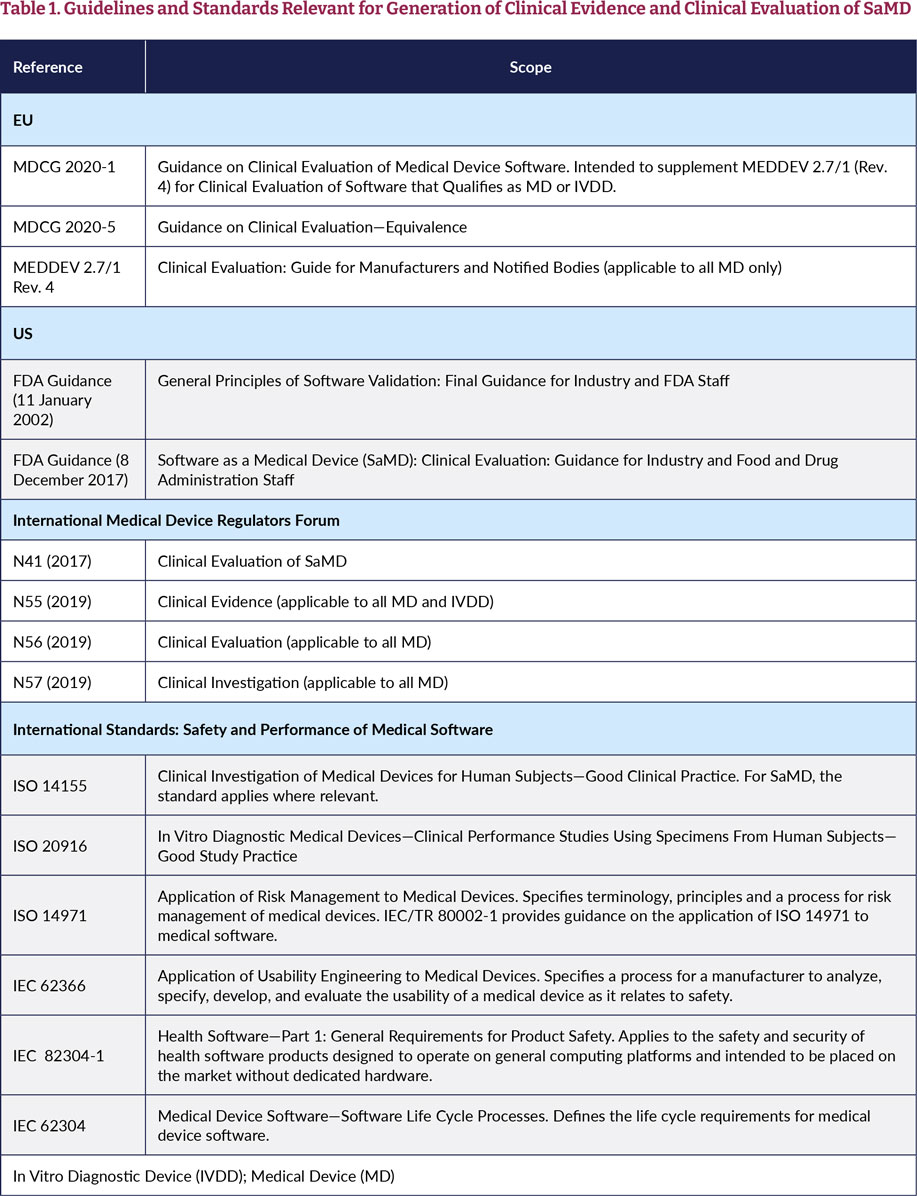
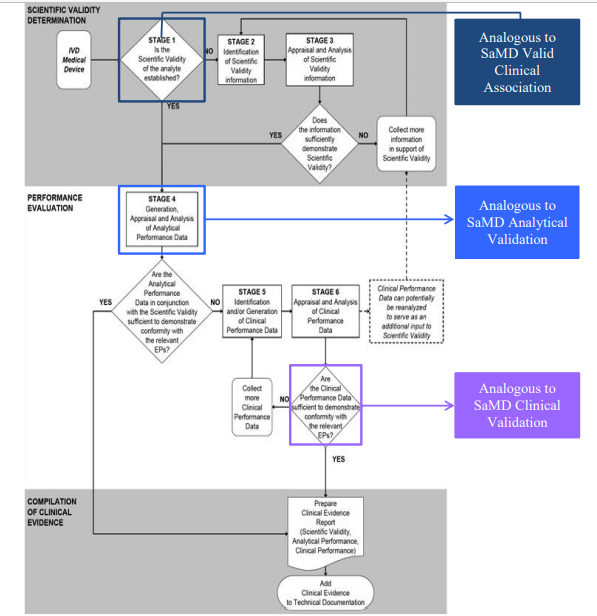
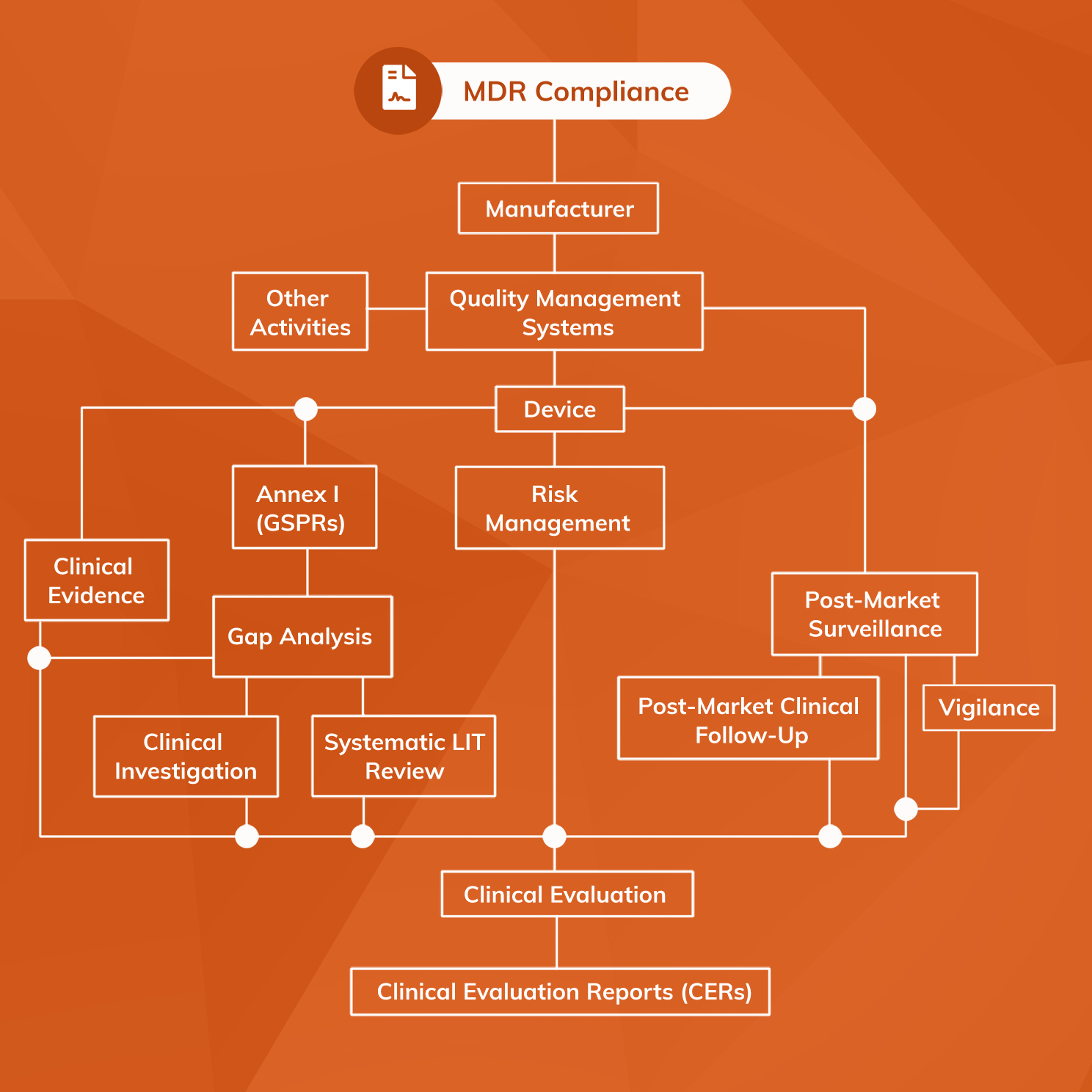
Software as a Medical Device (SAMD): Clinical Evaluation - Guidance for Industry and Food and Drug Administration Staff

Global Medical Device Podcast: What You Need to Know About Clinical Evaluation & Validation for Software as a Medical Device (SaMD)
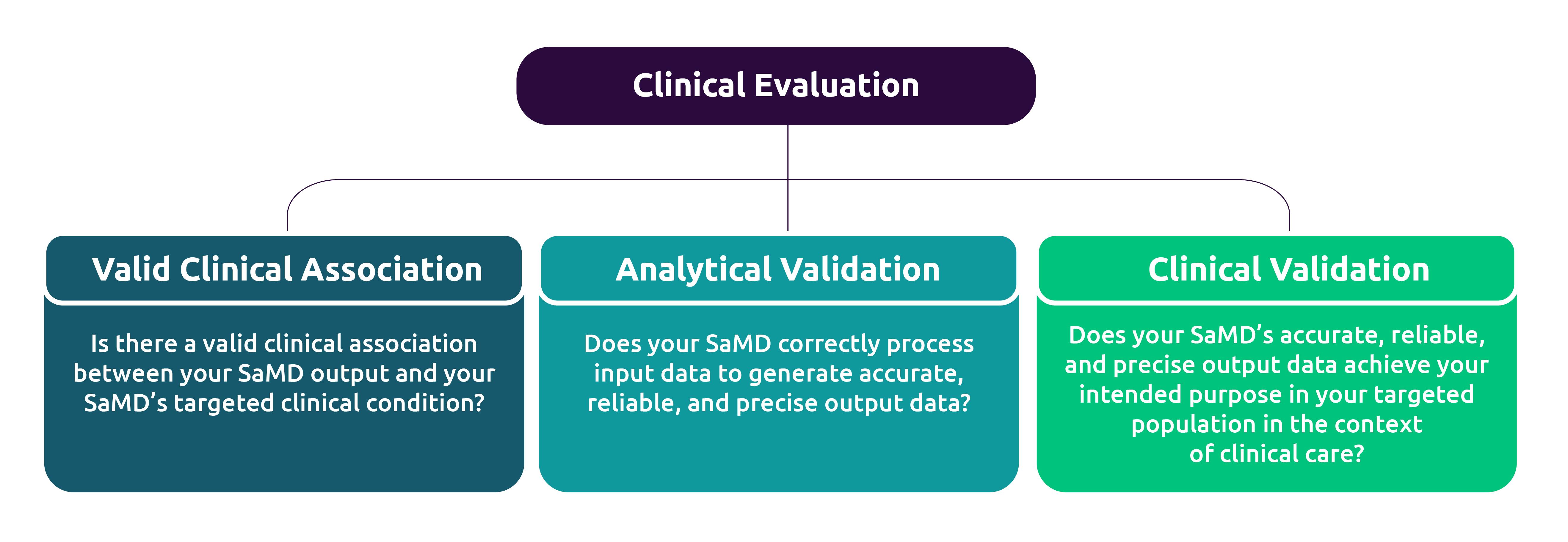
Verification and validation for next-generation healthcare – Software as a Medical Device (SaMD) | Capgemini